


- ISO 17025 2017 CHANGES ARCHIVE
- ISO 17025 2017 CHANGES ISO
Management Review Procedure (Clause 8.9).Internal Audit Procedure (Clause 8.8.2).Report of Customer Satisfaction (Clause 8.6.2).Addressing Risks and Opportunities Procedure (Clauses 8.5.2 & 8.5.3).You should also maintain any other documents and records that you have identified as necessary to ensure your management system can be maintained efficiently and improve over time, such as:
ISO 17025 2017 CHANGES ISO
Management Review Record (Clause 8.9.2)Ĭommonly used non-mandatory ISO 17025 documents and records. Audit Nonconformity Report (Clause 8.8.2d). Measurement Uncertainty Record (Clause 7.6.3). Complaint, Nonconformity and Corrective Action Report Log (Clause 8.7.3). Corrective Action Report (Clause 8.7.3). Sampling Report (Clause 7.3.3) (as applicable). Sampling Plan (Clause 7.3.1) (as applicable). Method Verification, Validation and Development Record (Clauses 7.2.1 & 7.2.2). Equipment Maintenance Record (Clause 6.4.13g). Calibrated Equipment Record (Clause 6.4.13a). List of Laboratory Equipment (Clause 6.4.13a). Record of Laboratory Environmental Controls (Clause 6.3.3). List of Approved Suppliers of Products and Services (Clause 6.6.2a). Supplier Evaluation and Approval Record (Clause 6.6.2a). Competence Approval and Authorization Record (Clauses 5.6 & 6.2.5e). Training Record and Performance Monitoring (Clause 6.2.2). Quality Objectives (Clauses 8.2.1 & 8.2.2). ISO 17025 2017 CHANGES ARCHIVE
Registry of Records for Detention/Central Archive (Clauses 8.3.2f & 8.4.1).List of Internal and External Documents (Clauses 8.2.4 & 8.3.1).Here are all the required records according to ISO 17025:2017: Records are generated to demonstrate compliance with the standard and related internal procedures and serve as evidence during audits. Calibration Report and Certificate Requirements Procedure (Clauses 7.8.2 and 7.8.4, applicable to calibration laboratories that write calibration certificates).
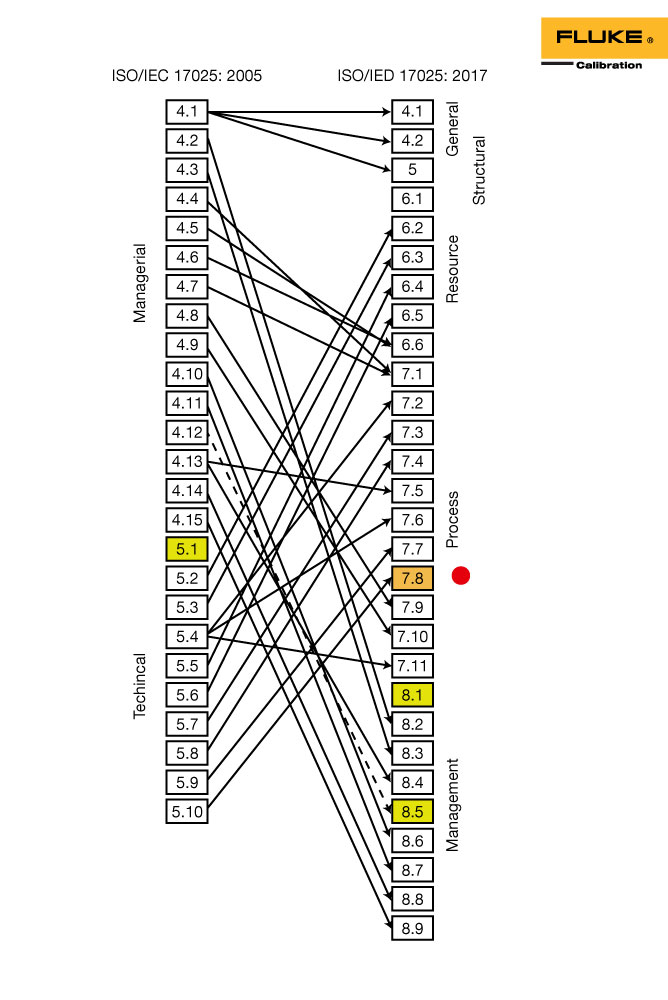 Testing Report Procedure (Clauses 7.8.2 & 7.8.3, applicable to testing laboratories that write test reports). Complaint, Nonconformity and Corrective Action Procedure (Clauses 7.9 – 7.10 & 8.7). Handling of Laboratory Test or Calibration Items Procedure (Clause 7.4). Sampling Procedure (Clauses 7.3, 7.5 & 7.8.5, applicable only to laboratories that do sampling). Quality Assurance Procedure (Clauses 7.7.1 – 7.7.3). Test and Calibration Method Procedure (Clauses 7.2.1 & 7.2.2). Customer Service Procedure (Clauses 7.1.1 & 8.6). Equipment and Calibration Procedure (Clauses 6.4.3 & 6.5). Facilities and Environment Procedure (Clause 6.3). Externally Provided Products and Services Procedure (Clause 6.6.2). Competence, Training and Awareness Procedure (Clause 6.2.5). Document and Record Control Procedure (Clauses 8.2.1, 8.3 & 8.4). Keep in mind that if you exclude some of the clauses from the scope of your implementation, then documents for those clauses will not be required for your lab. The documents listed below are must-haves according to ISO 17025:2017.
Testing Report Procedure (Clauses 7.8.2 & 7.8.3, applicable to testing laboratories that write test reports). Complaint, Nonconformity and Corrective Action Procedure (Clauses 7.9 – 7.10 & 8.7). Handling of Laboratory Test or Calibration Items Procedure (Clause 7.4). Sampling Procedure (Clauses 7.3, 7.5 & 7.8.5, applicable only to laboratories that do sampling). Quality Assurance Procedure (Clauses 7.7.1 – 7.7.3). Test and Calibration Method Procedure (Clauses 7.2.1 & 7.2.2). Customer Service Procedure (Clauses 7.1.1 & 8.6). Equipment and Calibration Procedure (Clauses 6.4.3 & 6.5). Facilities and Environment Procedure (Clause 6.3). Externally Provided Products and Services Procedure (Clause 6.6.2). Competence, Training and Awareness Procedure (Clause 6.2.5). Document and Record Control Procedure (Clauses 8.2.1, 8.3 & 8.4). Keep in mind that if you exclude some of the clauses from the scope of your implementation, then documents for those clauses will not be required for your lab. The documents listed below are must-haves according to ISO 17025:2017.







 0 kommentar(er)
0 kommentar(er)
